With high speed screw rod, thermoplastic material is continuously extruded in a tubular shape. Aseptic air blows to the plastic
tube parison, and the parison goes through the forming mould.
-
- English

| Quantity: | |
|---|---|







1150pcs/h
New
Automatic
Electric
380V/220V 50Hz
Liaoning, China
2021
Xianglong
4000*4000*6000mm
12000
2 years
High-accuracy
Ordinary Product
Provided
Provided
2 years
PLC, Motor
pharmaceutical factory
Japan, Germany, United Kingdom, Ukraine, Mexico, Spain, Pakistan
Filling Machine, AFP Blow-Fill-Seal Plastic (SVP)
Plastic
20-1000ml
Aseptic packing
PP/PE
9600 / 6000
Food, Beverage, MEDICAL, Chemical, Pharmaceutical
Bottles
Beer, Milk, Water, Oil, Juice, Pharmaceuticals
GMP ; IOS







| Model | Specification | No.of Extruding Heads | Distribution of Cavity | Total Cavities | Capacity(pcs/h) |
| AFPL6 | 500ml | 6 | 6 | 6 | 1150 |
| 1000ml | 6 | 6 | 6 | 1080 | |
| AFPL8 | 100ml | 8 | 8 | 8 | 1950 |
| 250ml | 8 | 8 | 8 | 1800 | |
| 500ml | 8 | 8 | 8 | 1550 | |
| AFPL6D | 500ml | 6 | 2*6 | 12 | 2350 |
| 1000ml | 6 | 2*6 | 12 | 2460 | |
| AFPL8D | 100ml | 8 | 2*8 | 16 | 4000 |
| 250ml | 8 | 2*8 | 16 | 3600 | |
| 500ml | 8 | 2*8 | 16 | 3100 |




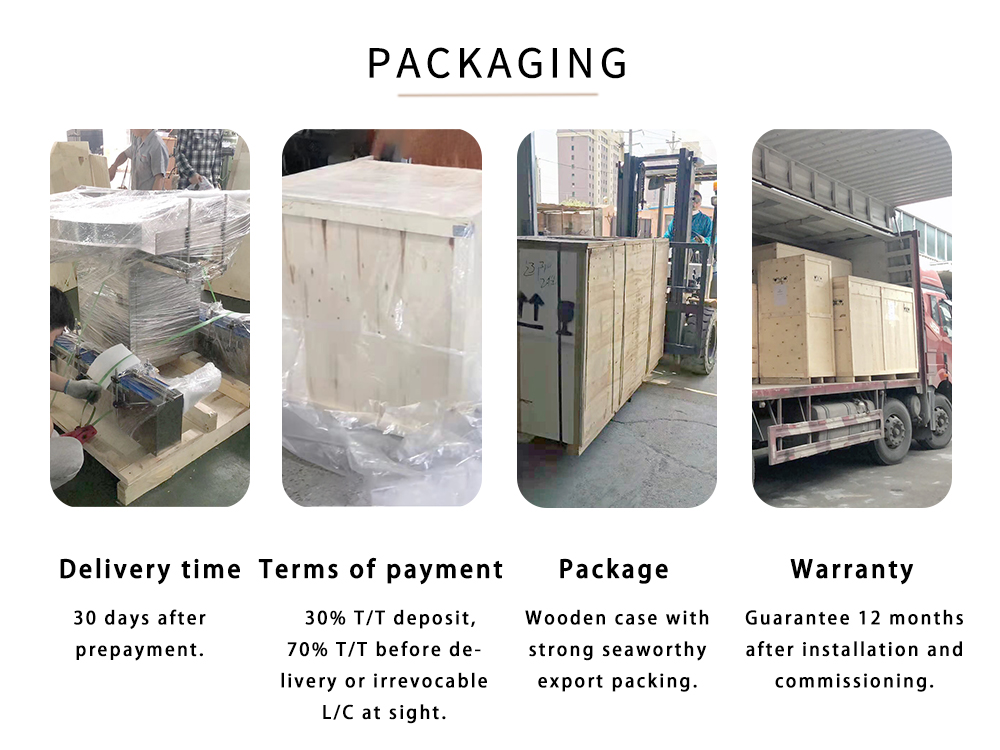












| Model | Specification | No.of Extruding Heads | Distribution of Cavity | Total Cavities | Capacity(pcs/h) |
| AFPL6 | 500ml | 6 | 6 | 6 | 1150 |
| 1000ml | 6 | 6 | 6 | 1080 | |
| AFPL8 | 100ml | 8 | 8 | 8 | 1950 |
| 250ml | 8 | 8 | 8 | 1800 | |
| 500ml | 8 | 8 | 8 | 1550 | |
| AFPL6D | 500ml | 6 | 2*6 | 12 | 2350 |
| 1000ml | 6 | 2*6 | 12 | 2460 | |
| AFPL8D | 100ml | 8 | 2*8 | 16 | 4000 |
| 250ml | 8 | 2*8 | 16 | 3600 | |
| 500ml | 8 | 2*8 | 16 | 3100 |














